Every month we share 2-3 write-ups on companies we decided to examine as potential additions to our portfolio. Although the companies analyzed may tick most of the boxes, if the margin of safety is not considered sufficient, we will not initiate a position but rather monitor the stock.
At the end of each write-up, we will state whether we decided to buy the stock or not. If not, keep an eye to our Quarterly Portfolio Update releases in which we will update you for all the transactions that took place during the latest quarter.
The Company in this week’s write-up is Novartis AG (Ticker: $NVS), a leading global medicines company.
“Today also is an important day. 25 years ago, Novartis was launched as a conglomerate, a broad conglomerate, not just in health care. Over the years, we were able to focus as a health care conglomerate. But today, we launched a strategy where we are transitioning to a fully focused innovative medicines company with the announcement to separate Aspen and Sandoz.” Vas Narasimhan - CEO
1. Key Facts
Description: Novartis AG (“Novartis”, “NVS”, and “Company”) was incorporated in 1996 and is a multinational group of companies specializing in the research, development, manufacturing and marketing of a broad range of innovative pharmaceuticals and cost-saving generic medicines (Sandoz Division). NVS employs c. 108,000 people and its products reach c. 800 million people globally.
Key Financials: Over the period 2012 to 9M 2022, the Company had a relatively flat revenue and operating income reaching a Trailing Twelve Month (“TTM”, “LTM”) revenue of c. $52.3B and an operating income of $9.8B (LTM margin of 19%). NVS has a healthy balance sheet with Cash and Short-term investments of $19.0B compared to a debt amounting to $26.8B (excluding leases).
Market Cap (as of 28th October 2022): Its market cap is $173.9B with a 52-week high of $94.26 and a 52-week low of $74.09, whereas it currently trades at $80.76, a 14.3% decline from its 52-week high.
Valuation: NVS trades at an EV/EBITDA of 11.9x (10 Year average of 14.7x) and at a TTM P/E of 8.2x (10 Year average of 22.6x).
2. Business Overview
Strategic Update
Novartis used to be a health care conglomerate but in the last few years took actions to transform the Company, such as selling its shareholdings in Idenix Pharmaceuticals and LTS Lohmann back in 2014, disposing its 36.5% shareholding in GSK Consumer Healthcare Holdings in 2018, spinning off Alcon Inc. (its eye care business) in 2019 and disposing its 33.3% shareholding (6.2% voting rights) in Roche Holdings on December 6, 2021.
Currently, the Company has two operating segments, namely, Innovative Medicines (“IM”) and Generics (Sandoz) contributing c. 80% and c. 20% of revenues, respectively. Recently, a strategic decision was taken to separate Sandoz from the Group moving along with a spin-off so as to transform Novartis to a pure-play Innovative Medicines company. Sandoz along with Teva Pharmaceutical comprise the 2 largest Generic Pharma companies worldwide.
“We will continue to deliver improved financials with +4% sales CAGR through 2027 and a Core Op Inc margin ~40%+ in the mid – long term. Our disciplined capital allocation will balance continued investment in the business and returning capital to our shareholders.” Vas Narasimhan - CEO
Novartis’ strategic focus will be on five therapeutic areas - cardiovascular, immunology, neuroscience, solid tumors and hematology, to become a top-5 player in US by 2027 (from #10) and top-3 in China (from #5) as well as to maintain its leadership in Germany (#1) and Japan (from #4 to #3) and to leverage on 3 emerging technology platforms i.e., gene & cell therapy, Radioligand therapy, and ‘xRNA’.
Technology: The Company is not new to these platforms, and it has the experience to capitalise on. For example, gene therapy – Zolgensma, cell therapy – KYMRIAH, Radioligand – Pluvicto (which recently obtained positive CHMP opinion of European Medicines Agency and was FDA approved in March 2022) and xRNA- Leqvio.
US: To achieve the US mind-set, the following actions are taken: US units reporting directly to Executive Committee, building U.S talent, increasing share of US patients in trials and to follow a US target product profile and to supplement it with other trials needed for EU compared to a historic global target profile.
Further to the potential spin-off of Sandoz, the new organisational model announced is expected to drive cost savings of c. $1.5B in Selling, General & Administration (“SG&A”) expenses by 2024, i.e., a c. 10% saving in SG&A which represents c. 3% of sales. This program comes with a one-time restructuring cost estimated to be 1.2x of this annual saving ($0.8B was already incurred year-to-date).
Key Products
In the below table we present the Top 20 revenue generating brands of NVS and highlight the 8 potential multi-billion sales winners identified by management.
Source: Novartis Annual and Quarterly filings, StockOpine analysis, Note: The green highlighted brands were not part of Top 20 portfolio in the specific period. cc stands for constant currency
As it can be observed from the above table, the top 10 brands follow a growth trajectory with the exception of Gilenya. Tasigna and Lucentis had slight declines (in constant currency) explained by lower sales in Europe and Japan with Lucentis impacted by the launch of biosimilars.
Despite the patent protection on Gilenya, the Company is having a hard time dealing with the entrance of Generics in US as the US Supreme Court lifted its prevention of generic versions of this multiple sclerosis drug entering the market on October 13, 2022. The patent on dosage regimen that was set to expire in 2027 was the one that was challenged and found invalid in US in Abbreviated New Drug Application (“ANDA”) proceedings filed by a generic product manufacturer. As a result, FDA-approved Gilenya generics were launched in US.
Per management earlier estimates, FY 2022 sales are expected to be negatively affected by $300M (incorporated in the updated 2022 outlook). As this is a highly margin product it can slightly impact margins in the near future but not the long-term target of +40% core operating margin by 2027 as generic entries were anyway expected in mid-2024 (per management).
All in all, this is the game for Innovative Medicine companies; developing blockbuster drugs through R&D, expand those to treat other indications, being able to protect rights as long as possible and to have an effective commercialisation strategy. For sure, recent developments regarding Gilenya is a cause for concern and is a short-term headwind for Novartis but that alone does not make Novartis less successful.
Potential winners
In respect to the 8 multi-billion brands, namely, Cosentyx (Immunology), Entresto, Leqvio (Cardiovascular), Zolgensma, Kesimpta (Neuroscience), Kisqali, Pluvicto (Solid Tumor) and Scemblix (Hematology) we should note that Management expects over $7B peak sales for Cosentyx (TTM revenue of $4.95B) and over $5B for Entresto (TTM revenue of $4.3B).
All of these brands are protected by patents. For example, considering both EU and US patents and including RPD (regulatory data protection), Cosentyx patents start to expire in 2026 with its latest patent expiring in 2033, Entresto (2023 until 2030), Zolgensma (2024 until 2033), Kesimpta (2023 to 2037), Kisqali (2028 to 2032 except one which expires on 2022) etc.
Few highlights for each brand:
Cosentyx (psoriasis, ankylosing spondylitis, psoriatic arthritis): plan to get to +$7B sales, however, to achieve that target an expansion in China is needed –> currently lockdown headwinds. Positive results from Phase 3 (SUNSHINE, SUNRISE) pivotal trials to meet the high unmet need of one of the most common dermatological condition (Hidradenitis Suppurativa) –> currently 95% are not on biologic treatment
Entresto (chronic heart failure): over 8m patients on therapy -> only 1/3 of eligible HFrEF (heart failure with reduced ejection fraction) patients are currently on treatment in the G7. There are ongoing litigations in China against generics and NVS believes it is fully protected for 2023 and 2024
Leqvio (LDL cholesterol): Adoption of healthcare professionals doubled in Q3’22 to 4,800 but adoption takes time and acceleration is expected in mid-2023. Targeted number of patients in US is 18M but NVS does not need a big share to achieve its financial goals (>$2B)
Zolgensma (Spinal muscular atrophy): Approved in 45 countries, negotiations for +10 countries, aim to increase newborn screening >35% in EU (in US is close to 98%) –> Intravenous indication alone can generate $1.5B-$2B annual sales
Kesimpta (relapsing remitting multiple sclerosis): Accelerating prescriptions with New to Brand prescriptions (“NBRx”) increasing 47% Vs a decline of 20% in the market
Kisqali: Metastatic breast cancer %age of NBRx increased from c. 10% in Sep-21 to 26% in Aug-22. NATALEE (early breast cancer) study continues -> Phase 3 readout expected in 2023 –> sales potential of >$3B
Pluvicto: 14% NBRx share in post-taxane mCRPC (metastatic castration-resistant prostate cancer), 120 treatment centers with a plan to get to 350-400, increasing manufacturing capacity, positive CHMP opinion of EMA
Scemblix: 13% patient share on total third-line setting (“3L”) & 39% NBRx across Chronic myelogenous leukemia (“CML”), accelerated approval converted to regular approval, rollout in EU following approval in Q3’22, Phase III for 1L ahead of plan and with >$2B potential
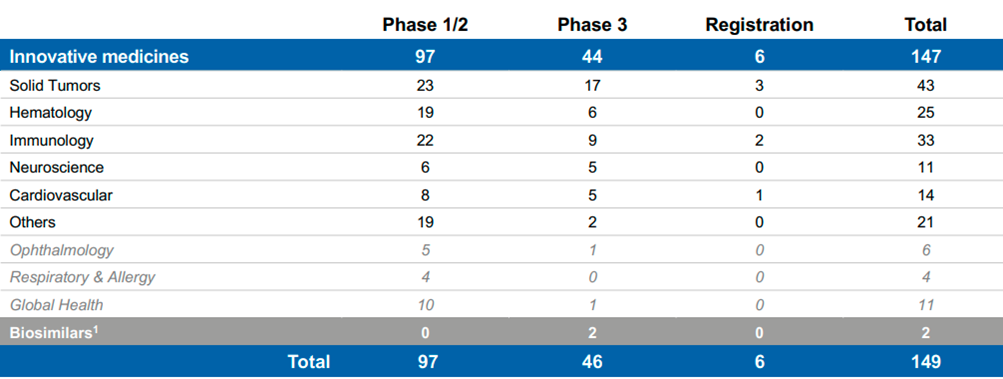
Pipeline
Novartis has a respectable pipeline at each therapeutic area at a different status/stage and potentially different peak sales estimates (as provided by management). Novartis has proved its ability to place new Innovative drugs/treatments in the market and appropriately commercialize those. The pipeline does not include only new brands but existing brands which are tested for treating other/similar diseases/indications, different age groups and reach other geographical areas.
Source: Novartis Q3’22 Results presentation, Pipeline projects at a glance
Management also disclosed in its R&D Day 2021 that there are 20 potential assets with >$1B sales potential with expected approval by 2026 (the 2026-2030 Growth Drivers).
Other drugs worth mentioning are, a) Iptacopan a drug with more than $3B sales opportunity (multi-blockbuster potential across indications) which recently met its two primary endpoints in Phase III trial showing superiority to previous treatment in adults with paroxysmal nocturnal hemoglobinuria (PNH – a rare, chronic and serious complement-mediated blood disorder) experiencing residual anemia despite prior treatment with anti-C5s and b) Remibrutinib chronic spontaneous urticaria Phase 3 REMIX-1 and -2 trials with expected readout in 2024 and more than $2B potential.
Pipeline is strong for the near future and there is a promising pipeline beyond 2030.
Key Geographies
Excluding Sandoz, as it is expected to be spun-off in mid-2023, the majority of Innovative Medicines Sales come from Europe (c. 36% in 2021, c. 33% in 9M 2022) and from US (c. 36% in 2021, c. 38% in 9M 2022). As there is no breakdown on a per country basis for Innovative Medicines but only on a Total basis (incl. Sandoz), we should also note that the US accounted for 33% of sales in 2021, Germany for 9%, China for 6% and both France and Japan for 5%.
Given the strategy ‘shift’ mentioned above, we should expect the US and China reliance to grow, while maintaining its position in EU.
We believe that the ‘shift’ to US makes sense considering that $589B (or 53%) of expected 2022 worldwide revenue in pharmaceuticals ($1,109B) is generated in US (Source: Statista).
3. Industry
Competition Landscape
A. Revenue
There are various companies who compete with Novartis for the same set of customers such as AstraZeneca, Bristol-Myers, Roche, Novo Nordisk, Sanofi, Abbott Laboratories, AbbVie, Pfizer, Eli Lilly, GlaxoSmithKline, J&J, Amgen and Merck & Co to name a few.
In terms of 2021 Revenue, Novartis is estimated to be the 5th largest Pharma Company, however, this is expected to change in the coming years following the spin-off of Sandoz (c. 20% of Novartis sales). Novartis used to be a larger player in the market (before the spin-off of Alcon, $7.1B revenues in 2018) but in the last few years is picking up through the expansion of its Innovative Medicine portfolio (Compound Annual Growth Rate (“CAGR”) of 6.8% from FY2017 to FY2021).
Source: Statista – Feb 2022 release, Leading global pharmaceutical companies based on pharma revenue in 2021 (in billion U.S. dollars)
Pfizer during 2021 had a significant impact from COVID-19 related revenues with Comirnaty (mRNA Pfizer vaccine) accounting for 45% of its revenue whereas Roche revenue was also impacted by Covid-Diagnostics tests accounting for 7.5% of its total revenue.
In relation to Abbvie, which is also larger than Novartis, a single immunology drug (Humira) accounts for c. 37% ($20.7B) of 2021 revenue. Nonetheless, its’ other two key immunology drugs (Skyrizi and Rinqov) are expected to reach $15B in sales (combined) by 2025 compared to $4.6B in 2021.
Although there is intense competition in the Pharma industry, new competitors are unlikely to enter or disrupt the key players given the large R&D investments (median 2021 -> 17.3% of sales) that these companies need to take to reach commercialization phase of a drug and the long approval (normal) times (from research to approval can take from 12-15 years). To get an idea of the FDA process you can read this article.
We assume that the key players will continue to play a key role in the industry and be the ones to drive the expected increase in Pharmaceuticals industry from $1,109B expected in 2022 to $1,431B by 2027 (CAGR of 5.2%).
B. Profitability
In general, the Pharmaceutical market is lucrative with high margins and free cash flow (“FCF”) conversion. Comparing the latest fiscal year of NVS with its peers, NVS lies somewhere in the middle with its EBITDA margin (33.9%) slightly below the median of its comparables (36.8%) whereas its FCF margin (25.9%) represents the median of the group.
We believe that NVS could improve its profitability positioning as it will spin-off Sandoz (a lower margin business, <20% EBITDA margin), due to the forecasted streamlining of expenses (saving of $1.5B p.a.) and the targeted core operating margin of +40% Vs an average 35% for Innovative Medicines during 2019-2021 (37.1% during 9M 2022).
Biosimilar/generic level landscape
Other than competition from peers who may develop alternatives or more efficient treatments, Generics and Biosimilars can hit the revenues of Novartis and other Pharma companies.
Further to Gilenya issues analysed earlier, looking at Novartis established medicines we identify in almost all brands (most notable being Afinitor/Votubia, Gleevec/Glivec and Exjade) a decline with the main reason being generic competition. Additionally, Abbvie’s Humira rest of world sales reported a decline in 2021 of 9.6% and 13.8% in Q2’22 with the main explanation being ‘Biosimilar Competition’. Similar explanations were provided for the drop in sales of J&J REMICADE drug ($3.7B sales in 2020 and $3.2B in 2021).
It shall be noted that Novartis is in ANDA litigation in US with generic manufacturers for Entresto ($3.4B sales in 9 months 2022), for Kisqali ($874M sales in 9 months 2022) and Xiidra ($342M sales in 9 months 2022), whereas Lucentis showed a minor decline in sales in the latest 9 months due to biosimilars impact in Japan.
4. Financials
NVS had a relatively stable Revenue over 2012-9M 2022 (“Historic Period”) with a similar trend in profits and free cash flows with an Operating Margin (“OPM”) of 19% and a FCF margin of 24%.
Source: StockOpine analysis, Koyfin
As stated above, the Company had significant strategic changes over the last years and the drop observed in 2017 and 2018 revenues is justified by the transfer of Alcon revenues of $6.8B and $7.1B, respectively, to discontinued operations. The key driver of revenue replacement/growth was the IM segment which grew from c. $32.3B in 2017 to c. $41.6B in LTM (CAGR of 5.51%).
No significant improvement is observed in OPM, however, since 2018 there is an upward trend from 18% to 22% in 2021, given the increasing weight of IM which carries a ten-year average OPM of c. 25% (LTM is 22.4% due to higher impairments and restructuring costs relating to the streamlining model). The drop in 2018 compared to 2017 was a result of high restructuring costs, whereas in 2019 and 2020, Sandoz put a downward pressure on margins due to significant impairment of Intangible Assets and a resulting segment OPM of 5.7% and 10.8%, respectively (average of 13.2% for the period 2012-2018 and 16.6% in 2021).
NVS will be able to achieve higher margins in the future given the potential spin-off of Sandoz segment and move closer to the long-term target of +40% Core Operating margin, well above the current 33.4% for 9M 2022 but not that distant to the 36.2% achieved for the IM segment in 2021 (9M 2022 stands at 37.1%).
Lastly, NVS has a solid financial position with Cash and Short-term investments of $19.01B compared to a debt amounting to $26.79B and leases of c. $1.75B, accompanied by strong credit ratings both from Moody’s (A1) and S&P (AA-) (of the highest compared to peers).
5. Capital Allocation
The policy of the Company is to invest organically through R&D and Capital Expenditures (“CAPEX”) with $9.9B and $1.3B spent in LTM, respectively (or 19% of revenue and 2.4%, respectively), to distribute dividends to shareholders with $7.5B returned to shareholders in 2022 (estimated yield 3.8%) to engage in value-creating bolt-ons (acquisitions) and to repurchase shares.
The Company is heavily involved in M&A transactions with Goodwill ($28.7B) accounting for c. 24% of its total assets and a total amount of c. $30B spent in acquisitions within 2017 to 2021 (per September call).
NVS initiated the payment of dividends in 1997 and increased the dividend per share (from 0.63CHF to 3.1CHF) consistently depicting a CAGR of 6.9%. Within 2017-2021, and including share repurchases, $53B were returned to shareholders (or c. 85% of FCF). As of 30 September 2022, $8.1B worth of shares were repurchased of which $7.2B relate to the $15B program announced in Dec 2021 following the sale of Roche stake. It seems that management follows an opportunistic approach in share repurchases rather than a structured repurchase program.
Vas Narasimham, CEO “And in terms of share buybacks, when we have excess capital that we're not able to deploy to our other priorities, we've been committed as well to doing that as appropriate with $15 billion of share buyback announced post the sale of the Roche stake. And still, more than half of that to be executed at a time period, we would have believed our relative share price is suppressed. We were able to sell an asset at a high and buy back our asset, our shares at a relative low.”
Although the Return on Equity of Novartis averages at 15% for the last 10 years (or 12.4% excluding 2021 when Roche stake was disposed) its Return on Capital (average of 6.9% in last ten years) is at the lower end compared to its peers and thus we cannot conclude that its capital allocation strategy is the most efficient in the industry.
6. Risks
Regulation governing Generics and biosimilars and patent vulnerability: As per Hatch-Waxman Act in US (similar laws in EU) eliminated the requirement for extensive clinical trials for generic manufacturers (as it is the case for the reference product) as long as it can be shown to be theoretically equivalent by filing an ANDA. As in the case with Gilenya, and the potential lost sales, Novartis is in ANDA litigation in US for Kisqali, Entresto and Xiidra and other drugs may face similar issues in the future. As Generics or Biosimilar launch can be detrimental to a drug’s value, weakness to defend patent validity (see Gilenya) can negatively impact future profits.
This is not a company specific risk but rather an industry wide risk with another recent example being the US Supreme court declining Biogen’s bid to reinstate its patent on its blockbuster multiple sclerosis drug Tecfidera against Viatris subsidiary.
FX continues to be a headwind: A strong USD negatively affects reported sales and core operating income. Assuming that October 2022 rates prevail, the impact on Sales and Core operating income for 2022 and 2023 are expected to be -7% and -8% for 2022 and -4% and -5% for 2023, respectively.
7. Outlook
For 2022, management expects IM sales growing mid-single digit and core operating income by mid-high single digits (including Gilenya impact). The improvement in margin will be driven by both the increase in sales and the cost saving program.
For Sandoz, management updated its guidance upwards to low-mid single digit (from low) for revenue and to low-single for core profit (from flat). As you will notice in the valuation section, we attributed a value of $18.3B to Sandoz. Such updates could result to a higher realised value upon spin-off.
The Company also expects to see financial expenses savings of $100M-$150M due to reinvestment of Roche proceeds and a core tax rate of 16.5%.
Long term wise NVS is expected to generate +4% CAGR in sales (IM) and a core operating margin of +40% including corporate costs.
8. Valuation
The stock price as of 28th of October 2022 stands at $80.76 and is down by 7.7% YTD. The market cap of the Company stands at $173.9B and trades at an EV/EBITDA TTM multiple of 11.9x. Based on our DCF valuation the estimated price of NVS stands at $92, 14% higher than the current price level, with a resulting IRR over a 5-year period of 14.2%.
Source: StockOpine analysis
To estimate the fair value (“FV”) of Novartis we employed a DCF approach for the Innovative Medicines segment and a Multiples approach for the Sandoz segment.
In respect to the Innovative Medicines segment, for the projection period we assumed a revenue CAGR of 2.5% which is in line with its 10-year CAGR of 2.7% but lower than its 5-year CAGR and Industry expected growth of 5.5% and 5.2%, respectively. Given the recent developments with Gilenya, the drastic impact that generics could have (see Afinitor/Votubia, Gleevec/Glivec and Exjade drop in 9M 2022) and the unfavorable currency rates we applied a gradual growth p.a. before reaching the +4% target of management.
In terms of profitability, we used an average projected EBITDA margin of 35.3% and a terminal EBITDA margin of 37.6%. Both average and terminal EBITDA margins are higher than the average EBITDA margin of the IM segment for 2019-2021 of 35.2%, however, our assumption accounts for the improvement in margins due to the cost savings/streamlining model (expected savings of $1.5B p.a.) and management’s expectation for a +40% Core Operating Margin including corporate costs.
To derive the free cash flows to the firm we deducted projected Capex requirements of 2.7% in line with its 3-year average.
In respect to the terminal EV/EBITDA multiple, it is assumed to be around 11.5x which is lower than its 5-year average of 15.7x and its current multiple of 11.9x. We consider this assumption fair as it is in line with its peers’ median of 11.6x and slightly lower than the average of 13.6x.
To calculate the value per share we used the minimum required return that we aim to obtain from our investments (i.e., 10%) under the current environment, adjusted for net debt and non-operating assets / liabilities identified and thereafter divided the resulting value by the number of shares.
Before dividing the value by the number of shares we also added the estimated fair value of Sandoz segment based on Multiples approach. Per our calculations, Sandoz FV was estimated at $18.3B based on a normalized EBITDA of $2.28B and an EV/EBITDA multiple of 8.0x. For comparison, Teva Pharmaceuticals (>50% revenue from generics) and Viatris (c. 30% revenue from generics) have a current and 5-year average multiples of 7.1x & 8.5x (Teva) and 6.2x & 8.9x (Viatris), respectively.
Based on the above calculations and assumptions used (which of course may not materialise at all), we reach a value per share of $92, 14% higher than the current price of $80.76 with a resulting IRR over a 5-year period of 14.2%.
Sensitivity analysis
The below table gives an indication of the potential upside/(downside) %age compared to the current price ($80.76 as of 28th of October) when the terminal multiple and the discount rate are changed.
Source: StockOpine analysis
9. Concluding Remarks
NVS operates in an industry with high barriers of entry that is ‘controlled’ by few key players.
We believe that NVS transition to a pure-play innovative medicine company, a more lucrative segment albeit riskier, the focus on US market and the concentration on 5 key therapeutic areas can contribute towards achieving higher returns than what was returned to its shareholders in the past years (total 10Y return of 92%). Additionally, its strong pipeline with 20 potential blockbuster drugs can drive its future and mitigate any negative impact from generics entry or biosimilars.
Given that NVS and pharmaceutical companies in general provide some form of protection to an economic slowdown and given its potential IRR of 14.2%, we believe that NVS makes a good fit to our portfolio. Therefore, we decided to initiate a position of 3.5% so as to follow its transition.
You can find us at Commonstock and on Twitter @StockOpine.
Disclaimer: The team does not guarantee the accuracy or completeness of the information provided in the newsletter. All statements express personal opinions based on own financial and business analysis. Any estimates or forward-looking statements made are inherently unreliable. No statement of opinion is an offer or solicitation to buy or sell the financial instruments mentioned.
The content of our newsletter is not a trading or investment advice and we do not provide any personal investment advice tailored to the needs of any recipient. The information provided should not be considered as a specific advice on the merits of any investment decision. Securities trading involve risk and you might lose your capital and/or incur other damages. Investors should make their own research and consult their registered investment advisor or financial advisor before taking any investment decision.
Neither the team nor any of its affiliates accept any liability whatsoever for any direct or indirect loss however arising, from any use of the information contained herein. Any unauthorized copy of this newsletter or its contents is illegal.
This post may contain affiliate links, which means that we might get a commission if you decide to sign-up using any of these links. No extra cost is charged to you.
By subscribing / reading our newsletter or any affiliated social media accounts, you indicate your unconditional acceptance to the above and your unconditional acceptance to our terms and conditions.









First of all, thank you for your work! I follow you on Twitter and plan to take some of your insights, as well as other Newsletters, as basis in order to structure my personal Company Analysis.
Just a quick question (even though this is an old thesis): if your estimated stock price is 14% higher than the stock price at that moment in time, how could the IRR in 5 years be as well 14%?
A yearly CAGR of 14.2%, starting at 81$, would give me a final price of around 157$ after 5 years. Am I missing something here?
Thanks in advance for your response.